Lyme Publications
Lyme Publications

P95 has collaborated in a large review study about Lyme disease. This study has involved databases/surveillance and systematic reviews focusing on Lyme disease incidence, epidemiology, hospitalization, and clinical manifestations, among other outcomes, in different regions of the World. Launched in 2020, the study involved many P95 employees and has now resulted in nine publications in a special issue of the Vector-Borne and Zoonotic Diseases Journal:
➡ Burn L, Vyse A, Pilz A, Tran TMP, Fletcher MA, Angulo FJ, Gessner BD, Moïsi JC, Stark JH. Incidence of Lyme Borreliosis in Europe: A Systematic Review (2005-2020). Vector Borne Zoonotic Dis. 2023 Apr;23(4):172-194. doi: 10.1089/vbz.2022.0070. PMID: 37071407; PMCID: PMC10122234.
➡ Nuttens C, Bessou A, Duret S, Skufca J, Blanc E, Pilz A, Gessner BD, Faucher JF, Stark JH. Epidemiology of Lyme Borreliosis in France in Primary Care and Hospital Settings, 2010-2019. Vector Borne Zoonotic Dis. 2023 Apr;23(4):221-229. doi: 10.1089/vbz.2022.0050. PMID: 37071406; PMCID: PMC10122229.
➡ Burn L, Tran TMP, Pilz A, Vyse A, Fletcher MA, Angulo FJ, Gessner BD, Moïsi JC, Jodar L, Stark JH. Incidence of Lyme Borreliosis in Europe from National Surveillance Systems (2005-2020). Vector Borne Zoonotic Dis. 2023 Apr;23(4):156-171. doi: 10.1089/vbz.2022.0071. PMID: 37071405; PMCID: PMC10122223.
➡ Paradowska-Stankiewicz I, Zbrzeźniak J, Skufca J, Nagarajan A, Ochocka P, Pilz A, Vyse A, Begier E, Dzingina M, Blum M, Riera-Montes M, Gessner BD, Stark JH. A Retrospective Database Study of Lyme Borreliosis Incidence in Poland from 2015 to 2019: A Public Health Concern. Vector Borne Zoonotic Dis. 2023 Apr;23(4):247-255. doi: 10.1089/vbz.2022.0049. Epub 2023 Jan 27. PMID: 37071404; PMCID: PMC10122228
➡ Houben E, de Jong H, Penning-van Beest F, Kuiper J, Holthuis E, Blum M, Skufca J, Riera-Montes M, Gessner BD, Pilz A, Vyse AJ, Begier E, Dzingina M, Herings R, Stark JH. Incidence of Lyme Borreliosis in the Dutch General Practice Population: A Large-Scale Population-Based Cohort Study Across the Netherlands Between 2015 and 2019. Vector Borne Zoonotic Dis. 2023 Apr;23(4):230-236. doi: 10.1089/vbz.2022.0048. Epub 2023 Jan 27. PMID: 37071403; PMCID: PMC10122225.
➡ Burn L, Pilz A, Vyse A, Gutiérrez Rabá AV, Angulo FJ, Tran TMP, Fletcher MA, Gessner BD, Moïsi JC, Stark JH. Seroprevalence of Lyme Borreliosis in Europe: Results from a Systematic Literature Review (2005-2020). Vector Borne Zoonotic Dis. 2023 Apr;23(4):195-220. doi: 10.1089/vbz.2022.0069. PMID: 37071401; PMCID: PMC10122246.
➡ Skufca J, De Smedt N, Pilz A, Vyse A, Begier E, Blum M, Riera M, Gessner BD, Stark JH. Incidence of Lyme Borreliosis in Finland: Exploring Observed Trends Over Time Using Public Surveillance Data, 2015-2020. Vector Borne Zoonotic Dis. 2023 Apr;23(4):256-264. doi: 10.1089/vbz.2022.0047. Epub 2023 Jan 27. PMID: 37071400; PMCID: PMC10122252.
➡ Skufca J, Tran TMP, Brestrich G, Pilz A, Vyse A, Malerczyk C, Dzingina M, Begier E, Blum M, Riera-Montes M, Gessner BD, Stark JH. Incidence of Lyme Borreliosis in Germany: Exploring Observed Trends Over Time Using Public Surveillance Data, 2016-2020. Vector Borne Zoonotic Dis. 2023 Apr;23(4):237-246. doi: 10.1089/vbz.2022.0046. PMID: 37071399; PMCID: PMC10122258.
➡ Nagarajan A, Skufca J, Vyse A, Pilz A, Begier E, Riera-Montes M, Gessner BD, Stark JH. The Landscape of Lyme Borreliosis Surveillance in Europe. Vector Borne Zoonotic Dis. 2023 Apr;23(4):142-155. doi: 10.1089/vbz.2022.0067. PMID: 37071402; PMCID: PMC10122255.
P95’s fundraising intiatives
/*! elementor - v3.11.5 - 14-03-2023 */
.elementor-widget-image{text-align:center}.elementor-widget-image a{display:inline-block}.elementor-widget-image a img[src$=".svg"]{width:48px}.elementor-widget-image img{vertical-align:middle;display:inline-block}
/*! elementor - v3.11.5 - 14-03-2023 */
.elementor-column .elementor-spacer-inner{height:var(--spacer-size)}.e-con{--container-widget-width:100%}.e-con-inner>.elementor-widget-spacer,.e-con>.elementor-widget-spacer{width:var(--container-widget-width,var(--spacer-size));--align-self:var(--container-widget-align-self,initial);--flex-shrink:0}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container,.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer,.e-con>.elementor-widget-spacer>.elementor-widget-container,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer{height:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner{height:var(--container-widget-height,var(--spacer-size))}
P95's Charity Donations

“From a deep respect for all human beings and our planet, we encourage and enable each other to thrive, learn and evolve” is part of P95’s purpose. As actions speak louder than words, from an internal fundraising initiative, P95 made multiple donations in 2023 that will help charities across the globe. The initiative, with a dual purpose of supporting our staff’s health and raise money for good causes, involved employees recording their physical activities in a company Strava club. For each hour of activity logged, the P95 management pledged to donate 2 euros to charity. This year, the money raised was donated to the following charities, chosen by P95 employees:
-
- Cool Earth: an organization that aims at helping rainforest communities, and funding projects that protect indigenous people impacted by the climate crisis. P95 believes the future of healthcare cannot be separated from the future of our planet.
More information on Cool Earth can be found here: https://www.coolearth.org/
- Cool Earth: an organization that aims at helping rainforest communities, and funding projects that protect indigenous people impacted by the climate crisis. P95 believes the future of healthcare cannot be separated from the future of our planet.
-
- Project for People: an organization that creates international projects in Italy, India, Brazil and Benin. P95’s donation helped the creation of the Nutritional Diet Project in India. This project aims at improving the living conditions of children in rural districts in West Bengal. With better tools, care and assistance this project combats malnutrition.
More information on Project for People can be found here: https://www.projectforpeople.org/
- Project for People: an organization that creates international projects in Italy, India, Brazil and Benin. P95’s donation helped the creation of the Nutritional Diet Project in India. This project aims at improving the living conditions of children in rural districts in West Bengal. With better tools, care and assistance this project combats malnutrition.
-
- Just Dig It: an organization that aims at cooling down the planet by restoring green spaces in Africa. So far, Just Dig It has restored around 300,000 hectares, and this amount keeps growing every day.
More information on Just Dig It: https://justdiggit.org/
- Just Dig It: an organization that aims at cooling down the planet by restoring green spaces in Africa. So far, Just Dig It has restored around 300,000 hectares, and this amount keeps growing every day.
-
- Lesoochranárske zoskupenie VLK: an organization that protects Slovak forests from deforestation. P95 supported their “Buy Your Own Tree” initiative that will help the organization acquire and protect more land.
More information on Lesoochranárske zoskupenie VLK can be found here: https://www.wolf.sk/en/en-home
- Lesoochranárske zoskupenie VLK: an organization that protects Slovak forests from deforestation. P95 supported their “Buy Your Own Tree” initiative that will help the organization acquire and protect more land.
Humanity and health are at the center of P95’s values. By helping charities and organizations, we hope to make the world a better place, not only for the future but also for the present.
LOGEX and P95 to jointly build the international infectious disease observatory ARWEN ID
LOGEX and P95 to jointly build the international infectious disease observatory ARWEN ID

Healthcare analytics provider LOGEX and P95, a company specialized in epidemiology of infectious diseases, have signed an agreement to jointly create an international infectious disease observatory. The goal of the observatory is to offer an international platform for research of infectious diseases like COVID-19, RSV, Influenza, Norovirus and other infections that may have a significant health impact in Europe. Additionally, the platform will be used for assessing the impact, effectiveness and safety of vaccines or other pharmaceutical health interventions.
The observatory will initially include 20 hospitals in five European countries. The first proof-of-concept studies will be presented at the end of 2023. The observatory’s name, ARWEN ID, is derived from the Actionable Real World Evidence Network (ARWEN) and its focus on infectious diseases (IDs).
Find us on LinkedIn to follow the progress of this new joint effort.



Official Opening of P95 Thailand
Official Opening of P95 Thailand

Almost two years after expanding its operations to Latin America with the creation of P95 Latina, our company has announced the creation of a new branch office in South East Asia (SEA) based in Thailand. In this interview, Tharinee Sakhakorn, Regional Director for SEA shares a bit more about the experience of setting up this subsidiary, the main challenges of the process and the objectives and reasons to choose Thailand as the main hub for the office.
Please tell us about your background and how you came to meet P95.
Tharinee Sakhakorn: I joined the pharmaceutical industry almost 20 years ago and was very fortunate to have the opportunity to bring an international component to my career. I have had a variety of roles in research and development at the global, regional and country levels, including project management, training, quality risk management, change management and strategic leadership. I worked for GSK Vaccines (Thailand and Belgium), Takeda Vaccines (Switzerland), Roche (Thailand) and Clover Biopharmaceuticals prior to joining P95 as a Regional Director for SEA.
I am passionate about developing talent, transforming and leading the growth of the business. So, when I heard about P95’s potential expansion to SEA, I was very motivated by the opportunity to make an impact on the organization and contribute meaningfully to the region.
How has the process of setting up the subsidiary been? What have the main challenges been?
TS: It is generally quite easy to set up a company in Thailand as a Thai Limited Company. However, we have made the decision to get P95 SEA registered to the Board of Investment (BOI) as a Contract Research Organization (CRO), which is a little complex and time-consuming. It involves compiling a variety of corporate documents and supporting materials, including a detailed business plan and financial plan. Our application was accepted in the first round and our interview with the BOI officials went quite smoothly (thanks to our CEO Thomas Verstraeten’s detailed explanation of our services to help the BOI understand what P95 has to offer).
The main challenges have been following through different steps, getting the required documents together and ensuring that we are fulfilling different criteria for BOI and the Department of Business Development (DBD) to be fully operational.
What was the reason to choose Thailand as the main hub for the SEA region? What is the landscape of epidemiology and research in this country?
TS: We looked at three factors when assessing the entity location for P95 SEA, including the research landscape, the talent pool and the business environment.
Thailand has a high incidence of tropical diseases, including dengue fever, hepatitis, malaria, Japanese encephalitis and rabies. Among the SEA countries, Thailand has the highest number of studies listed on ClinicalTrials.gov (more than 3,200 studies and about 530 of these are observational) and the highest number of publications on epidemiology.
Thailand has a significant presence of research units with foreign collaborations, international pharmaceutical companies and CROs. Epidemiology experience is mainly found in academic and post-graduate settings, but is still quite limited in the industry (which we saw as an opportunity). In the subject of life sciences and medicines, Thai universities are among the top 10 in SEA.
With its strategic location in the heart of mainland SEA, Thailand is well connected and safe and has long been recognized as an attractive place to live. It is an easier place to do business, and overall, it has a great potential for epidemiology research.
What are the goals for the SEA office in the short term? How about the longer term?
TS: In order to establish and grow our presence in the region, we will first focus on gaining insights and understanding the needs of the pharmaceutical industry, academia, organizations and other CROs for a complete view of our customers. We will also prioritize customer collaboration and engagement through different channels and experiences. At the same time, we aim to form and develop a high-performing team to offer project delivery excellence and high-quality execution.
Our ultimate goals are to develop a business, increase our pipeline and collaboration opportunities in the region and build bridges between regional and international epidemiological and clinical research networks that eventually support access to vaccines and medicines.
P95 held its annual retreat in Mallorca
P95 held its annual retreat in Mallorca
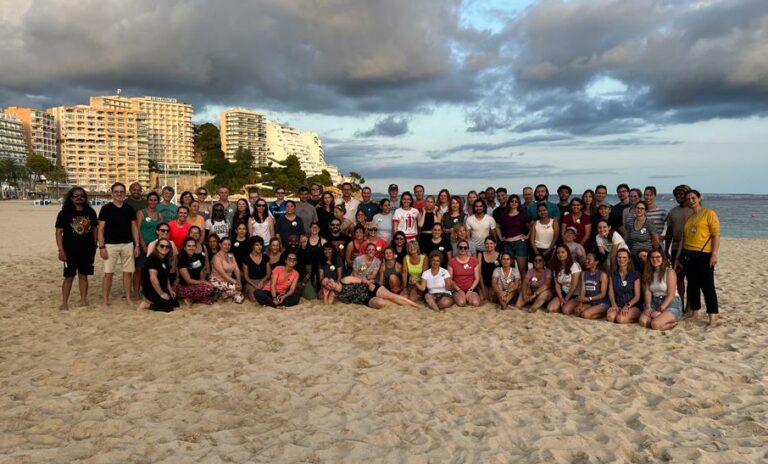
At the beginning of October, we held our Annual Retreat in beautiful Mallorca. Around 100 employees were present! This was a great opportunity to network, meet our colleagues, discuss past, present and future of P95, acknowledge our diversity, and party together. What a wonderful couple of days!
A tour of our new P95 Latina office
A tour of our new P95 Latina office
We're excited to share a quick tour of our new P95 Latina office in Bogotá, Colombia, which opened in August 2022. The office provides a tailored space for our local team to meet, work together, or share a coffee. This is a new step toward strengthening our Latina branch office, a little over a year after its creation, and we hope our colleagues will make the most of it.
P95 presents 5 posters at the International Conference on Lyme Borreliosis 2022
P95 presents 5 posters at the International Conference on Lyme Borreliosis 2022

The P95 Lyme Team (Leah Burn, MPH, Jehidys Montiel Ramos, Aura Victoria Gutiérrez Rabá, Thao Mai Phuong Tran) presented 5 posters at the International Conference on Lyme Borreliosis (ICLB 2022) in Amsterdam, the Netherlands from Sept. 4th to 7th.
The posters were:
[P027] Incidence of Lyme Borreliosis in Europe from National Public Health Surveillance Systems (2005-2020)
[P028] Worldwide epidemiology of Lyme of disease outside the regions of North America, Europe, and China: A systematic review (2005-2020)
[P029] Incidence of Lyme Borreliosis in Europe, a systematic literature review (2005-2020)
[P030] Seroprevalence of Lyme Borreliosis in Europe: results from a systematic review (2005-2020)
[P047] Incidence of Lyme Disease in Asia from National Public Health Surveillance Systems (2011—2020)
P95 authors are on 6 total poster presentations at ICLB2022
P95 featured in the GIZ’s 2021 Integrated Company Report
P95 featured in the GIZ’s 2021 Integrated Company Report

The Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH's 2021 Integrated Company Report highlights the international training program to enhance vaccine pharmacovigilance capacity in low and middle-income countries developed last year as a joint effort between P95 and GIZ. Through this initiative, our company contributed its knowledge and expertise on pharmacovigilance and vaccine safety and effectiveness studies, while GIZ provided support to roll out of the training through an international network, which included health agencies and experts from Ecuador, Ghana and Morocco.
You can find more on this initiative, featuring our team lead Zuleika Aponte (she/her/ella/elle), here: https://reporting.giz.de/2021/our-work-around-the-world/global-health/greater-vaccination-safety
DRIVE project comes to an end
DRIVE project comes to an end
After five years, the Development of Robust and Innovative Effectiveness (DRIVE) project has come to an end. Set up in July 2017, DRIVE’s main goal was to establish a sufficiently sized network for robust, high-quality, brand-specific IVE estimates for all vaccines used in the EU in each season. After the project's end, all the partners involved expect its legacy to continue through the development of a vaccine monitoring framework in the EU.
P95 was an active consortium partner in DRIVE, leading the work on the annual influenza vaccine effectiveness studies, performing the statistical analysis of the data and authoring the annual reports. In addition, the P95 IT-team developed a GDPR-compliant secure environment for repository of the sites' datasets and for data analysis. P95’s participation was led by Anke Stuurman.
DRIVE formed the basis for COVIDRIVE, a brand-specific COVID-19 vaccine effectiveness platform, co-founded and co-coordinated by P95.
P95 celebrates its 11th birthday
P95 Celebrates its 11th birthday

On June 16h, 2022, P95 turned 11 years old! To everyone who has allowed our company to achieve this milestone, thank you! We are so excited to celebrate one more year doing what believe in: finding, analysing and reporting epidemiological data to help health agencies, research organisations and pharma companies in their quest to improve people’s access to safe and effective vaccines. We are proud of the achievements that the past 11 years have brought and look forward to the future. Here’s to many more years working together, new wonderful projects and contributions to public health.









