P95 Latina team meets in Bogota
P95 Latina team meets in Bogota

On the week of May 16th to May 20th, some of the Epidemiology and Medical Writing team leads met the P95 Latina team in Bogota for a productive work week, which also included presentations from each team member sharing their experiences in different P95 projects and, of course, some quality in-person time together. The team also took this opportunity to celebrate the first P95 Latina anniversary, and we hope this was the first of many more celebrations of our company's growth in other parts of the world.
“We saw in Latin America a hub of talented professionals in epidemiology and research”
“We saw in Latin America a hub of talented professionals in epidemiology and research”

Expanding to a different region is the dream of any growing company. For P95, the opportunity arose last year, when Adriana Bastidas, Regional Scientific Director and Blanca Escobar, Regional Study Operations lead, took on the challenge of creating P95 Latina, the first branch office of P95 outside Europe. Based in Colombia, where an important hub for clinical research and epidemiology in the region was identified, P95 Latina started operations in April 2021 and now counts with nine epidemiologists and one medical editor. In this interview, Adriana and Blanca explain the process of creating P95 Latina, its challenges, and its future.
Q: Why did P95 decide to expand to Latin America?
A: There were parallel interests that came together and created a perfect “symbiosis.” On the one hand, P95 had the idea of expanding to another geographic location to increase the geographic capacity in terms of projects and human resources. On the other hand, as Latin Americans with 20+ years of work experience in Europe, we wanted to take the skills and knowledge acquired to train people there and at the same time create work and development opportunities in the region, as well as link talented professionals with international epidemiological and clinical research companies and networks.
The idea of establishing a branch office in Latin America was a combination of both interests, and the decision to do it in Colombia was because we identified it as an important hub of talented professionals in epidemiology and public health. Clinical research centers in Colombia have shown significant development in the past 15 years and have become important for studies in Latin America and there is an open spirit to learn and further develop in epidemiology and clinical research. Also, Colombia has a large population (≈50 million people), regulations facilitate the establishment of foreign companies, and the country has had a very stable economy for more than two decades.
Q: How has the process of creating a new hub on the other side of the world been?
A: We started working with research centers and universities, which has been very interesting and enriching. It helped us confirm what we knew about Colombia: it is a country with a lot of interest in creating knowledge and professional networks. The researchers, professors, and coordinators have been very open and receptive to the idea and facilitated the process. In fact, they are very interested in a continuous collaboration.
Opening the branch in administrative and legal terms was also relatively easy. With the help of some personal contacts in Colombia and the Flanders Investment & Trade organization (FIT), we were quickly connected to several Colombian organizations, which facilitated many administrative aspects and decisions. Furthermore, Colombians are quite receptive towards development and business.
Q: What has been the most difficult and the most rewarding aspect of this process?
A: We have felt very welcome. The most rewarding thing has been identifying and creating a work team with very capable, committed professionals with a thirst for knowledge and progress. It has been especially gratifying to see how the group has integrated into P95 in such a short time, despite the distance and the challenges it poses. Also, it is encouraging to see all that has been done for research and training in epidemiology and clinical research in Colombia, resulting in leading study centers that know very well what they do (with international standards). There are many collaboration opportunities not only in the country but also in Latin America.
As there is so much potential for collaboration, perhaps a nice challenge rather than a difficulty (we see it as a luxury problem) is to identify the most efficient way to integrate local and regional project opportunities into the company's short-term global strategy.
Q: How do you see the near future for the Latin American hub? Are there plans to expand to other countries in the region?
A: The hub in Colombia is called P95 Latina because, since its creation, the intention has been to have this location as a center for Latin America. The near future includes increasing professional capacity, deploying more epidemiological studies in the region, and creating new collaborations with universities and research centers in Colombia and across the entire region.
“I want people to know that P95 has an important role in the improvement of public health worldwide”

Ten years after being founded and two years after experiencing an unprecedented growth that even led to its expansion in Latin America, P95 received its first nomination to the Trends Gazelles. This award recognizes the fastest-growing companies in Belgium on the basis of their growth in three areas: added value, staff, and cash flow. For Tom Verstraeten, CEO of P95, this nomination “means that the growth we’ve experienced in the past year is becoming visible at the public level, which is really good.”
Indeed, visibility is one of the benefits that the Trends Gazelles nomination brings to the company. According to our CEO, “this also makes P95 more attractive for potential investors and clients that may want to do business with us. I now get emails from people saying, ‘I see you’ve been listed; do you need a board of directors? Do you need a commercial officer? I can help’. Now, more people can see that we are doing well and that we’re not just any company. P95 will feature in a long list with other successful companies.”
From the business perspective, adds Tom, getting to know some of the companies on the list of nominees brings an opportunity to learn more about what other firms outside our network are doing. “I looked up a few of the other companies on the list and some of them have developed very rapidly. Among those, you see quite a few IT companies that develop software for very specific businesses. You also find companies that create a business out of things you wouldn’t have thought of. We have limited interaction with other sectors, but this nomination gives us access to other people and allows us to learn from what they do and how they make their businesses grow.”
For those who are finding out about P95, Tom wants them to know that “this is a young and innovative company, scientifically driven, and a good place to work, because we are competitive on the job market, and this is a place where people can develop themselves and gain experience. Finally, I hope people see that we have an important role in the field of public health, that we are contributing to the improvement of public health all over the world. Yes, it’s a huge world and we are just one player, but we are doing our part, just like other players are doing theirs.”
According to Trends Gazelles, the nominated companies are not only a “source of energy to the economy” but also an “inspiration to other entrepreneurs”. For those who might need inspiration, P95’s CEO shares his advice, “if you’re willing to work hard, you’ll make it. This is what I always tell people who seek my advice on how to start work on their own. There’s work to be done everywhere, and there’s a shortage of hard-working, well-intended, qualified people. But you also need flexibility. When I started, I thought that it would be all about pharmacovigilance, but then I realized that there was a bigger need in epidemiology. Many people, like me, have an idea and think it is a critical need, but then it turns it’s not exactly what you thought it needed to be, so you need to be flexible. You have to trust yourself and know that if you do good work, there’s going to be plenty of work for you.”
COVIDRIVE welcomes three new vaccine companies to the consortium
COVIDRIVE welcomes three new vaccine companies to the consortium
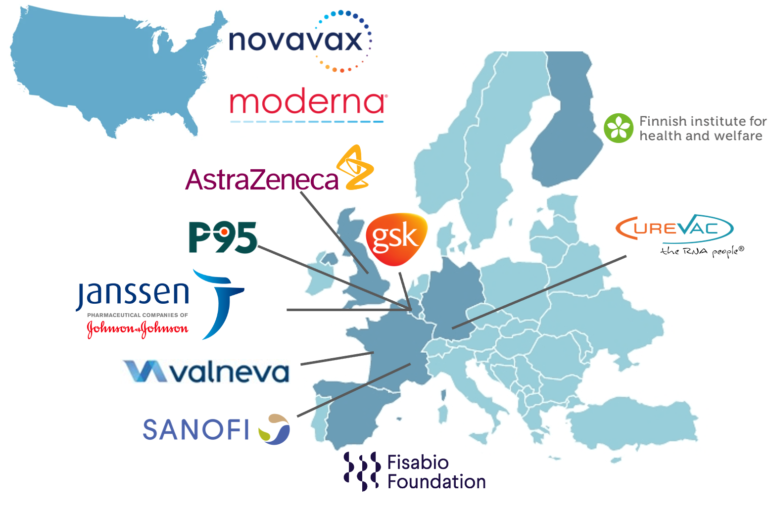
Moderna, Novavax and Valneva have joined COVIDRIVE, a public-private partnership for COVID-19 vaccine effectiveness monitoring in Europe.
08/02/2022 – Three new vaccine companies (Moderna, Novavax and Valneva) have joined the COVIDRIVE consortium and officially become COVIDRIVE partners. Moderna, Novavax and Valneva will further contribute to the assessment of the effectiveness of COVID-19 vaccines in Europe to support the region’s public health response and address the vaccine companies’ regulatory obligations.
With the inclusion of these three newcomers, COVIDRIVE, which brings together public institutions, small and medium-sized enterprises and vaccine companies, has a total of 11 partners.
The new COVIDRIVE partners reflected on their participation in the consortium and the value of multi-stakeholder initiatives to monitor the effectiveness of the COVID-19 vaccines.
First, Dr Jacqueline Miller, Moderna’s Senior Vice President and Therapeutic Area Head, Infectious Disease Development, explained: “We are pleased to join the COVIDRIVE consortium and contribute to this collaboration advancing global public health. We believe that public-private partnerships play a key role in combatting the COVID-19 pandemic. As a global company, it is critical to understand data from all geographies because as we have seen throughout this pandemic, what affects one part of the world, can soon affects others. Assessing vaccine effectiveness through real-world evidence is an important tool as we try to remain ahead of the virus as it evolves.”
Moreover, Dr Seth Toback, Senior Vice President, Global Medical Affair at Novavax, commented: “Novavax is proud to join COVIDRIVE and partner across the healthcare sector in our efforts against COVID-19. We believe it is important to have multiple vaccine options available for people, particularly as we assess the need for boosters, annual revaccination, and reaching the paediatric population. This is an exciting development as we continue to work towards the development of life-saving vaccines and fight against the ongoing pandemic.”
Finally, Dr Judith Perez Gomez, Valneva’s Vice-President of Medical Affairs pointed out that “Public-private partnerships have proven to be extremely valuable for enabling R&D innovation and are even more important today as we fight the COVID-19 pandemic. We are extremely pleased to join COVIDRIVE and support Europe’s public health response through the assessment of vaccine effectiveness.”
About Moderna
Moderna is a biotechnology company based in Cambridge, Massachusetts (United States) pioneering messenger RNA (mRNA) therapeutics and vaccines. The Moderna COVID‑19 vaccine, codenamed mRNA-1273 and sold under the brand name Spikevax, received the emergency use authorisation by the U.S. Food and Drug Administration (FDA) in December 2020 and conditional marketing authorisation in the European Union by the European Medicines Agency (EMA) in January 2021. More information: www.modernatx.com.
About Novavax
Novavax, Inc. (Nasdaq: NVAX) is a biotechnology company that promotes improved health globally through the discovery, development and commercialization of innovative vaccines to prevent serious infectious diseases. The company's proprietary recombinant technology platform harnesses the power and speed of genetic engineering to efficiently produce highly immunogenic nanoparticles designed to address urgent global health needs. NVX-CoV2373, the company’s COVID-19 vaccine, has received authorization from multiple regulatory authorities globally, including Conditional Marketing Authorization from the European Commission and Emergency Use Listing from the World Health Organization. The vaccine is also under review by multiple regulatory agencies worldwide. More information:www.novavax.com.
About Valneva
Valneva is a specialty vaccine company based in Saint-Herblain (France) focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need. Valneva has developed an inactivated, adjuvanted vaccine candidate against COVID-19, VLA2001, which is currently under rolling review by the EMA. More information: www.valneva.com.
About COVIDRIVE
COVIDRIVE is a public-private partnership launched in 2021 to address the joint need to monitor COVID-19 vaccination programmes for public health institutes in Europe and to assess brand- specific COVID-19 vaccine effectiveness (CVE) for the companies as part of their regulatory obligations. For more details on COVIDRIVE please check our website www.covidrive.eu.
If you would like to request a COVID-19 vaccine effectiveness study and/or participate as a partner/study site in COVIDRIVE, send an email to the following address: info@covidrive.eu
P95 nominated for the Trends Gazelles Flemish Brabant 2022
P95 nominated for the Trends Gazelles Flemish Brabant 2022

P95 is one of the nominees for the 2022 edition of Trends Gazelles Flemish Brabant. Trends Gazelles award aims to distinguish the fastest-growing Belgian companies over the past 5 years.
For 21 years, Trends Gazelles (www.trendsgazellen.be) has nominated between 50 to 250 companies in each province, distinguished as a source of energy for the economy and as inspiration for entrepreneurs. Nominees in 3 categories – small, medium, and large enterprises – are then ranked based on growth in added value, staff, and cash flow. They must have created at least 10 jobs since its creation and show sufficient operational independence. The 3 top-ranked companies, to be announced in February 2021, will be awarded the title of “Trends Gazelles Ambassador”.
P95 is honoured to have earned this recognition that shows the rising impact of the company at the regional and national levels.
P95 appoints Henk Hoornaert as Chief Commercial Officer
P95 appoints Henk Hoornaert as Chief Commercial Officer

P95 has appointed Henk Hoornaert as Chief Commercial Officer (CCO). After two years of accelerated growth, this appointment will strengthen P95’s structure and improve business development, marking a further step towards a more stable and mature organization.
Henk Hoornaert holds a Master in Physical Education and a Postgraduate degree in Economics from the University of Leuven. He developed a vast career in the pharma sector, from medical representative in 1992, to product manager, local and then regional marketing team lead for Pfizer’s portfolios, including Cardiovascular, Alzheimer and Inflammation. During the last 3 years, Henk has been General Manager, first in Belgium and Luxembourg for Upjohn, a Pfizer division, and later in Austria and Switzerland at Viatris.
P95 CEO Thomas Verstraeten reacts: “As we are nearing the end of 2021, we have made good progress in strengthening P95, and we have the opportunity to invest in improving our structures and hiring for new positions. Needless to say that Henk knows the pharma industry inside-out and his vast commercial and managerial experience will strengthen the management team significantly. As CCO, Henk will help us set up a commercial framework to support our ever-growing range and number of projects.”
Largest ever Lassa fever study expands to more countries in West Africa
Largest ever Lassa fever study expands to more countries in West Africa

July 28 2021, Oslo Norway, Cotonou Benin, Conakry Guinea, Monrovia Liberia, Abuja Nigeria, and Kenema Sierra Leone – The largest ever study created and funded by the Coalition for Epidemic Preparedness Innovations (CEPI) to provide a more accurate assessment of the incidence of Lassa fever infections has launched in several more countries in West Africa.
CEPI is providing US$ 10.3 million in funding to partners in Benin, Guinea, Liberia, and Sierra Leone to participate in the epidemiological research programme Enable, which will enrol up to 23,000 participants, including Nigeria, which began collecting participant data in December 2020. The in-country partners selected for the research are Fondation pour la Recherche Scientifique (FORS) in Benin, Phebe Hospital in partnership with the National Public Health Institute of Liberia (NPHIL) in Liberia, supported by University of North Carolina at Chapel Hill (UNC), Kenema Government Hospital in Sierra Leone (KGH) in cooperation with Tulane University, and Université Gamal Nasser de Conakry (UGANC) in Guinea, in partnership with Robert Koch Institut (RKI).
First identified in 1969, Lassa fever is a potentially deadly haemorrhagic illness occurring across West Africa, with an estimated 1% of cases proving fatal. It is listed on the World Health Organization (WHO) R&D Blueprint as an emerging infectious disease in urgent need of research and development and is also recognised in CEPI’s ambitious $3.5bn plan to tackle future epidemics and pandemics caused by known and unknown threats.
However, our current knowledge on the annual Lassa case rate is hindered by a lack of formal and standard clinical diagnoses for the illness and significant variability and severity in symptoms, with the majority of patients who become infected thought to be asymptomatic and failing to seek diagnosis. Cases may also occur in remote regions where there are difficulties in accessing health care services for testing. As a result, the true case count is unknown and likely to be much higher than current estimates of 100,000 to 300,000 cases per year.
The Enable study therefore aims to better understand the rate, location, and spread of Lassa virus across the region. Data collected in the countries will highlight any differences in the age and gender of people who become infected, while also providing a more accurate overview on the proportion of asymptomatic and symptomatic cases.
In addition, results from Enable will be crucial in supporting CEPI’s goal, as part of its five-year lookahead strategy, of producing a licenced Lassa vaccine for routine immunisation. As a leading funder of Lassa vaccine development, CEPI has already supported the development of six Lassa vaccine candidates. Two of these vaccines, developed by partners Inovio and Themis Bioscience, entered Phase I trials in 2019, and a further vaccine candidate, developed by IAVI, started in-human testing this year.
Data provided from the Enable research programme may therefore guide the location and implementation of future late-stage efficacy trials to evaluate these or other Lassa vaccine candidates. It could also help to define an appropriate vaccination strategy once a Lassa vaccine is approved for use, for example by helping to identify priority populations at risk.
The research will also support another goal in CEPI’s five-year plan assisting countries with developing the infrastructure and expertise to undertake the epidemiological and clinical studies needed to advance vaccine development and enable such countries to take full ownership of their national health security.
There have been a number of positive developments in the Lassa vaccine space over the past couple of years, with multiple candidates now moving into in-human testing. To continue this momentum and to meet CEPI’s goal to get a licensed Lassa vaccine for routine immunisation, we must therefore now advance our disease-assessment efforts to provide the critical data for future late-stage Lassa vaccine clinical trials.
The results produced by the Enable research programme will be vital in contributing to this endeavour, while also providing novel information to help support healthcare workers and researchers in the region working on this potentially deadly threat.
Melanie Saville
Director of Vaccine Research and Development, CEPI
Enable research partners will collect data on potential infections either through ‘active case follow-up’, whereby a field worker carries out repeat health assessments on study participants through home visits or phone meetings to assess the health status of participants, or ‘passive case detection’, where participants will be encouraged to report potential illness or to self-present at a health facility and the suspected or confirmed case is then recorded.
A subset of those enrolled will also take part in an additional assessment to look at the prevalence of Lassa fever antibodies–biomarkers of the immune response–among participants. This will act as an indicator to better guide estimates of how many individuals in the general population are likely to have previously been infected with the virus and are, at present, protected against (immune from) the disease; it is generally assumed that a single infection with Lassa fever virus will produce life-long protective immunity.
Enable research partners will collect data on potential infections either through ‘active case follow-up’, whereby a field worker carries out repeat health assessments on study participants through home visits or phone meetings to assess the health status of participants, or ‘passive case detection’, where participants will be encouraged to report potential illness or to self-present at a health facility and the suspected or confirmed case is then recorded.
A subset of those enrolled will also take part in an additional assessment to look at the prevalence of Lassa fever antibodies–biomarkers of the immune response–among participants. This will act as an indicator to better guide estimates of how many individuals in the general population are likely to have previously been infected with the virus and are, at present, protected against (immune from) the disease; it is generally assumed that a single infection with Lassa fever virus will produce life-long protective immunity.
I am delighted to be part of the Lassa fever study in West Africa. This rigorous scientific study will allow us to collect precise data to allow the country and partners to plan evidence-based interventions and controls.
Prof. Ayola Akim Adegnika
Principal Investigator, FORS Benin
The CEPI Enable epidemiological study is the largest Lassa haemorrhagic fever research project ever implemented in Guinea. In terms of public health, it will be of great interest both for the community to better understand the illness and for health authorities to appreciate the burden of Lassa fever. The community is grateful to CEPI for its support.
Dr. N’Faly Magassouba
Principal Investigator, Gamal Abdel Nasser University of Conakry, Guinea
There were challenges when we first started, and bottlenecks we’ve encountered, especially encouraging people in our culture to participate in research studies.
But our team has been working to enroll participants and we’re learning lessons as we go; in fact, as we go, our study is progressing better than we could have imagined. Our participants have expressed the pride they have in Liberia, and in their communities, for playing a role in a future that could ultimately be Lassa free.
Dr. Jefferson Sibley
Phebe Hospital Medical Director, Liberia
CEPI’s program to assess the incidence of Lassa virus infections in West Africa has been meticulously planned. These essential epidemiological studies will provide the foundation for clinical trials of promising Lassa vaccines that have to potential to achieve control of this massive public health threat.
Dr. Robert Garry
Tulane University, co-principal investigator working in cooperation with Kenema Government Hospital in Sierra Leone
Seven thousand participants have already been recruited into the Nigerian component of the Enable programme, led by the Nigeria Lassa fever Research Consortium consisting of the Nigeria Centre for Disease Control (NCDC) and supporting partners.
As part of CEPI’s access commitments, data for both the Nigerian study and programmes announced today will be made available to partners and the public via peer-reviewed open-access publications and via CEPI’s hub on The Global Health Network.
The Enable Lassa programme is building on collaboration among countries in West Africa most affected by Lassa fever to generate data and evidence that we urgently need for vaccine R&D. We are excited about the prospects of the project, not only in generating data for vaccine development, but also in strengthening our knowledge of the epidemiology of this disease and improving on the much-needed interventions for disease control.
Dr. Chikwe Ihekweazu
Director-General of Nigeria Centre for Disease Control
The study methodology and protocol were devised in collaboration with the coordinating partners who are now supporting the participating countries during implementation. All will follow a harmonised core protocol and methods to allow for standardised recording and comparability of data across the countries. Coordinating institutions supporting the overall Enable programme implementation include P-95, Margan Clinical Research Organization (MMARCRO), Epicentre, and the Bernard Nocht Institute for Tropical Medicine (BNITM).
The COVIDRIVE project has been officially launched
The COVIDRIVE project has been officially launched
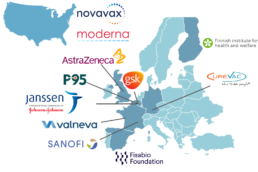
A new public-private partnership – COVIDRIVE – announced today that it will begin studies to assess the effectiveness of multiple COVID-19 vaccines in Europe to support the region’s public health response and to address the vaccine companies’ regulatory obligations. This multi-stakeholder partnership brings together public institutions, small medium enterprises and vaccine companies, including FISABIO (Spain), P95 (Belgium), THL (Finland), AstraZeneca (UK), CureVac (Germany), Janssen (Belgium), Sanofi-Pasteur (France) and GSK (Belgium).
The partnership will conduct several studies to analyse COVID-19 vaccine effectiveness in real-world conditions to complement what is known from robust clinical trials conducted for marketing authorisations. In addition to overall effectiveness for each brand of vaccine, key areas of interest include: duration of vaccine protection, effectiveness against disease caused by newly emerging SARS-CoV-2 strains, effectiveness against severe COVID-19 disease and effectiveness in special risk groups such as immunocompromised, frail individuals or subjects with chronic conditions or existing comorbidities.
COVIDRIVE will leverage an existing influenza vaccine effectiveness platform (DRIVE), which has provided annual brand-specific influenza vaccine effectiveness estimates to the European Medicines Agency (EMA) since 2017.
AstraZeneca and Janssen will be the first pharmaceutical companies in the partnership to launch their brand-specific COVID-19 vaccine effectiveness assessments through COVIDRIVE, starting in July 2021.
For more details on COVIDRIVE please check our website https://covidrive.eu
If you would like to request a COVID-19 vaccine effectiveness study and/or participate as a partner/study site in COVIDRIVE, send an email to COVIDRIVE Coordination team (info@covidrive.eu)
P95, Leuven-based company specialized in epidemiology and pharmacovigilance, acquires Rotterdam-based Pallas health research and consultancy (Pallas).
P95, Leuven-based company specialized in epidemiology and pharmacovigilance, acquires Rotterdam-based Pallas health research and consultancy (Pallas).
P95, Leuven-based company specialized in epidemiology and pharmacovigilance, acquires Rotterdam-based Pallas health research and consultancy (Pallas).
Thomas Verstraeten, CEO of P95: Thanks to our extensive expertise in the development and implementation of vaccines, we experienced a sharp increase in projects and associated growth during the COVID-19 pandemic. To allow us to further support the growing market of vaccination, we are looking for partners to sustain our growth. Pallas is a company with very similar expertise and clientele to P95. Judith van den Bosch and her team have developed a well-functioning and profitable company, close to the values of P95. This acquisition fits perfectly into our strategy to become an even more relevant player in the growing vaccination market.
Judith van den Bosch, founder of Pallas: Merging with P95 is a logical step in the development of Pallas. The types of projects and employees of Pallas and P95 are very similar, but the two companies also complement each other in terms of the research areas and customers. By becoming part of P95, I expect to be able to offer my team more opportunities and, together with P95, to be able to increase our impact clout now and in the future.
About P95
P95 was founded in 2011 by the current CEO, Thomas Verstraeten, former Vice President of the vaccine department and head of pharmacovigilance and epidemiology at GSK. In 2021, P95 has become an important player on the international stage of vaccine development, working for almost all major vaccine producers and international institutions such as the World Health Organization, the Gates Foundation and the European Commission. With a team of epidemiologists, statisticians, medical writers and analysts, P95 conducts epidemiological studies to help clients plan the development of new vaccines and monitor the effects of vaccines on the market. P95 also contributes to the development of COVID-19 vaccines and follow-up of their effectiveness after approval. (www.p-95.com)
About Pallas
Pallas health research and consultancy BV was founded in 1999 by Judith van den Bosch. Over the past 20 years, Pallas has become an established player on the contract research market in the field of epidemiology and health research. Pallas’ team consists of 12 epidemiologists with expertise in various disease fields and research methods. Pallas is based in Rotterdam, but has an international customer portfolio with a wide variety of organizations from the health sector and pharmaceutical industry. Pallas has been recognized in recent years for conducting systematic literature research in various contexts, such as clinical guidelines or drug development. (www.pallas-healthresearch.com)
P95 appoints Stefaan as Chief Financial Officer
P95 appoints Stefaan as Chief Financial Officer.

P95, a service provider specialized in epidemiology, statistics and pharmacovigilance, is happy to announce that Stefaan will be joining the management team as Chief Financial Officer, effective July 6th this year.
Stefaan is a senior executive with a proven track record in leading fast-growing companies. He previously held CFO positions at MobileXpense and Luciad, two belgian PE backed software companies. He began his career as an financial auditor at PwC.
Stefaan is a qualified accountant and holds a Masters’ degree in Applied Economics.
Thomas Verstraeten, managing director of P95 said ‘I’m very excited to have Stefaan join our management team. Stefaan brings us a wealth of experience in supporting SMEs like ours to achieve steady growth. Since we started 9 years ago we have seen our turnover, number of staff, diversity of expertise and scope of work significantly increase. Stefaan will be key in supporting us further on this growth trajectory and enabling us to better serve our ever increasing number of clients.’ Stefaan stated, ‘I look forward to joining P95 and leading its finance organization to contribute to P95’s future success as the company is rapidly scaling its operations.’
About P95.
P95 is a service provider, headquartered in Leuven, Belgium with staff in various European locations. Specialised in the epidemiology of infectious diseases and vaccine development, P95 supports public health agencies, vaccine manufacturers and others in setting up, conducting and analysing epidemiological studies and real world data as well as pharmacovigilance related activities. P95 is part of several Innovative Medicines Innovation (IMI) projects, including DRIVE (www.drive-eu.org) and VITAL (www.vital-imi.eu) and has recently won an award for 196 thousand Euros from VLAIO to support its development of artificial intelligence to better interpret real life data in support of vaccine development. P95 staff have co-authored over 65 peer reviewed publications.