P95 Expands its Global Presence with a New USA Office
P95 Expands Its Global Presence with a New USA Office
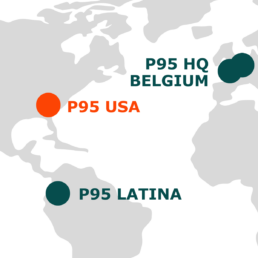
P95, a leading global provider of clinical and epidemiology services focused on vaccines and infectious diseases, has opened its first office in the USA. Based in the Research Triangle Park in North Carolina, the largest research park in the USA, this expansion underlines P95s commitment to delivering high-quality research solutions to our clients and partners across the globe.
The North Carolina USA office joins P95’s regional hubs in Europe, Africa, Southeast Asia and Latin America with over 200 experts in clinical operations, epidemiology, pharmacovigilance, data management, and biostatistics working together across continents and time zones. Together they will continue to provide first-class customized solutions covering clinical studies phase I-IV, epidemiology and RWE, and scientific and clinical development consulting.
"This is a significant milestone in our growth and development as a global contract research organization. This expansion will allow us to better serve our clients and partners in the region, as well as to leverage the opportunities and resources that North Carolina offers as a leading biotech and pharma hub. We look forward to building long-term relationships and delivering excellence in research in the USA and beyond." – Thomas Verstraeten, P95 Chief Executive Officer
P95 is proud to be a truly global CRO, with the ability to offer high-quality services tailored to the local needs and regulations of each country where it operates. P95’s commitment to excellence in research is driven by its passion for vaccines and infectious diseases and its dedication to advancing public health worldwide.
More about P95's full services
P95’s Journey From Epidemiology Consulting to Comprehensive CRO
P95's Journey From Epidemiology Consulting to Comprehensive CRO
In a recent interview for Ampersand Capital Partners, our CEO, Thomas Verstraeten, and Chairman, Benoit Bouche shared pivotal insights into P95's growth from an epidemiology consulting into a global comprehensive Contract Research Organization (CRO).
Almost one year from P95's partnership with Ampersand Capital Partners, the article dives deeper into P95’s expanding global reach, client-focused capabilities, and commitment to global health that bring our company to the forefront.
"In addition to COVID, there is an astounding need for vaccines to treat infectious diseases on a global scale, such as RSV, influenza, HMPV, and fast-spreading Dengue in and around South America. The demand has no limit, and P95 is ready to rise to the challenge and meet this need." - Thomas Verstaeten, P95 CEO
“Large-scale CROs often lack the necessary specialization and agility required to support the intense demand for clinical vaccine development. P95, with its reputation for deep expertise and flexibility in providing customized client services, stands out in this landscape." – Benoit Bouche, P95 Chairman

About Ampersand Capital Partners
Founded in 1988, Ampersand is a middle market private equity firm with more than $3 billion of assets under management dedicated to growth-oriented investments in the healthcare sector. With offices in Boston, MA and Amsterdam, Netherlands, Ampersand leverages a unique blend of private equity and operating experience to build value and drive long-term performance alongside its portfolio company management teams. Ampersand has helped build numerous market-leading companies across each of the firm’s core healthcare sectors.
P95 welcomes John O’Brien as Chief Business Officer
P95 welcomes John O’Brien as Chief Business Officer

We are excited to welcome John O’Brien as our new Chief Business Officer! John has a proven track record of driving commercial success and building strategic partnerships, bringing a wealth of experience to our team.
"I am delighted to welcome John to our team! His visionary leadership and innovative approach will be instrumental in driving our company forward as we continue our jounrey to be the premier provider of clinical and epidemiological services for vaccine developers worldwide." - Thomas Verstraeten, P95 Chief Executive Officer
John joins us from his previous role as Chief Commercial Officer at Excelya Group, a contract research organization (CRO) providing full-service clinical, FSP, and resourcing solutions. Prior to this, John held leadership and business development roles in various clinical CROs including Cmed, Chiltern, Theorem Clinical and Parexel.
Please join us in extending a warm welcome to John! We are excited to have him on board and look forward to his valuable contributions to our organization's continued success.
Stay tuned for exciting updates as we embark on this new chapter together!
COVIDRIVE expands its scope of studies and becomes id.DRIVE: new European partnership for burden and effectiveness studies on infectious diseases
id.DRIVE is a Public-Private-Partnership, which consists of a unique consortium of pharmaceutical companies, public health institutes and research groups who are joining forces to monitor infectious diseases and effectiveness of related pharmaceutical products in real-life in Europe. This is achieved by several pharmaceutical companies co-funding a research network of hospitals.
id.DRIVE leverages the COVID-19 vaccine effectiveness (VE) platform, COVIDRIVE (https://covidrive.eu/) (2021-2023) and Influenza VE platform, DRIVE (https://www.drive-eu.org/) (2017-2022). The partnership decided to make use of the established consortium and study network to expend its scope to all infectious diseases (IDs), lending its name to the new partnership. Next to vaccines, other pharmaceutical products such as antivirals and monoclonal antibodies will be studied.
While id.DRIVE will continue performing COVID-19 effectiveness studies, the consortium will initiate a first surveillance study starting mid-2024 on many other respiratory pathogens such as Influenza, RSV, Adenovirus, Human enterovirus, Parainfluenza virus, etc. This surveillance will help to understand the impact of these respiratory pathogens on serious illness, and to monitor the effectiveness of pharmaceutical products which are or will be available to prevent and cure these viral illnesses.
The id.DRIVE public-private partnership includes 8 partners, with AstraZeneca, Janssen, GSK, Novavax, Valneva and Pfizer being pharmaceutical company partners, P95 a Small and Medium Enterprise and Fisabio, a Spanish regional public health institute. The id.DRIVE study network covers 13 European countries and comprises 32 Study contributors, 5 primary care networks, and a nation-wide register. id.DRIVE is open for new partners to join the partnership and is expanding its study network.
To join us as id.DRIVE partner or study site, contact us at info@idDRIVE.org.
Find out more on www.iddrive.eu
P95 Attends National Clinical Research Network (NCRN) in Thailand in 2023

P95 Attends National Clinical Research Network (NCRN) in Thailand in 2023
[07/12/2023]
In November, P95 took center stage at a pivotal event in Thailand's clinical research scene—the National Clinical Research Network (NCRN) Conference 2023. Held in the vibrant city of Bangkok, this conference was a collaboration between NCRN, the Pharmaceutical Research and Manufacturers Association of Thailand (PReMA), and the national management unit for enhancing the country’s competitiveness (PMUC).
At this gathering, we connected with key opinion leaders (KoLs) who offered invaluable insights into the future of clinical research in Thailand. P95 seized the opportunity to expand our network and establish new partnerships.
For more information about the conference, please get in touch with our commercial team at P95 South East Asia Office.
Tharinee Sakhakorn (Regional Director, SEA)
https://www.linkedin.com/in/tharineesakhakorn/
Vaccination Programmes | Epidemiology, Monitoring, Evaluation book now available open access
Vaccination Programmes | Epidemiology, Monitoring, Evaluation book now available open access
Our P95 colleague Kaatje Bollaerts is excited to announce that the book ‘Vaccination Programmes | Epidemiology, Monitoring, Evaluation’ by Susan Hahné, Kaatje Bollaerts, and Paddy Farrington is now available to all to view and use online through a new open-access grant provided by CEPI.
Aimed at those involved, or planning to be involved, in many aspects of vaccination programmes, including public health professionals and epidemiologists, the book explores epidemiologic methods that can be used to study, in real life, their impacts, benefits, and risks.
Please feel free to download the book or read the book online through this link: https://lnkd.in/dAh65S2A
OnQ joins the P95 family

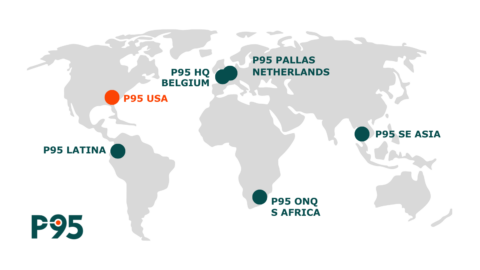
October 16, 2023. Leuven, Belgium and Johannesburg, South Africa - P95 BV (“P95”), a portfolio company of Ampersand Capital Partners and leading provider of clinical and observational services to vaccine developers, has signed an agreement to merge OnQ Research (“OnQ”) into P95. The transaction, which is subject to regulatory approval and scheduled to close before year end, furthers P95’s goal of becoming the leading global, vaccine-focused CRO. P95 now has unparalleled expertise conducting research in emerging geographies on the infectious diseases which could possibly drive a future pandemic. The combined company will also continue to offer clinical research services in disease areas beyond its vaccine focus (e.g. oncology, medical devices, cardiology).
Upon completion of the merger, the combined P95-OnQ will have a physical presence in over 30 countries with offices in Europe, Latin America, Asia, and Africa. Its services will cover both regulatory- grade real-world evidence and full-service interventional clinical trial research. Over the last 20+ years, the respective expertise of both organizations has been applied at the cutting edge of vaccine research for both bacterial and viral diseases including Influenza, Covid-19, Group B Strep, RSV, Hepatitis variants, and E Coli.
“This is an exciting transaction for our respective teams and clients, and an important step towards our goal of being the preferred service provider to all vaccine stakeholders,” said Thomas Verstraeten, CEO of P95. “The enhanced geographic footprint of the combined company will accelerate recruitment timelines, while providing our sponsor partners access to a global, diverse patient base. Further, the expansion into Africa for P95’s ongoing real world evidence projects provides a pathway to generate unique insights for both R&D prioritization and the monitoring of public health decision effectiveness.”
Catherine Lund, CEO of OnQ, said “We have grown exponentially in recent years and have proudly built a company whose quality standards are on par with those seen at North American and European CROs. Africa has enormous potential to contribute to global infectious disease research, and we believe merging with P95 will help accelerate the continent’s impact on the field. Together we will implement state-of-the-art clinical trial infrastructure and help establish the combined P95-OnQ as the unrivaled African champion in infectious disease clinical research services. Additionally, we see strong cultural alignment between the two companies to pursue sustainable economic empowerment which addresses the socioeconomic disparities present in the regions in which we operate.”
Benoit Bouche, Chairman of the Board at P95 and an Operating Partner at Ampersand Capital Partners, added, “I am proud to support a company with such high potential for positive contribution to the global struggle against infectious disease. We will pursue our mission with the ultimate goal of improving global access to effective vaccines.”
About P95
P95 is a global company headquartered in Leuven (Belgium) and with local and regional offices located in Rotterdam (the Netherlands), Bogota (Colombia) and Bangkok (Thailand). P95 delivers cutting-edge expertise in epidemiology and pharmacovigilance. The P95 team is comprised of epidemiologists, pharmacovigilance specialists, biostatisticians, data scientists, medical writers, IT engineers and study managers, located in over 17 countries. P95 projects are largely focused on a broad range of infectious diseases and vaccines, and the company also offers customized services upon request. Additional information about P95 is available at www.p-95.com.
P95 Attends Infectology Congress in Buenos Aires
P95 BV Attends Infectology Congress in Buenos Aires

Last week, P95 attended for the first time, the Congreso de Infectología 2023 organized by Asociación Panamericana de Infectología (API) and Sociedad Argentina de Infectología (SADI), which took place in Buenos Aires, Argentina.
This congress gathered renowned experts from the field of infectology, who shared lessons learnt and debated the most pressing and current topics on this topic.
More about the congress: https://www.congresosadi.com/
P95 Present at Bio Asia Pacific
P95 Present at Bio Asia Pacific

P95 is proud to be an official exhibitor at this year's Bio Asia Pacific in Bangkok. Bio Asia Pacific is one of the leading conferences and exhibitions for Biotechnology, Life Sciences and Smart Health in Southeast Asia. This year we had the opportunity to present a bit more about our company and our recently created Sout East Asia office in Bangkok by our Regional Director South East Asia, Tharinee Sakhakorn.
Extensive experience in the conduct of and support to epidemiological studies. Our activities include:
- Non-interventional studies on: burden of disease, safety, effectiveness, healthcare resource utilization, and others
- Primary data collection
- Feasibility
- Protocol development
- Investigators identification
- Data capture, transfer and storage
- Data and site monitoring
- Analysis and reporting
- Secondary use of electronic health record (EHR) databases, and access to a wide range of databases on demand
- Feasibility and fit-for-purpose assessment
- Protocol development
- Analysis and reporting
P95 BV and Ampersand Capital Partners
/*! elementor - v3.12.2 - 23-04-2023 */
.elementor-widget-image{text-align:center}.elementor-widget-image a{display:inline-block}.elementor-widget-image a img[src$=".svg"]{width:48px}.elementor-widget-image img{vertical-align:middle;display:inline-block}
/*! elementor - v3.12.2 - 23-04-2023 */
.elementor-column .elementor-spacer-inner{height:var(--spacer-size)}.e-con{--container-widget-width:100%}.e-con-inner>.elementor-widget-spacer,.e-con>.elementor-widget-spacer{width:var(--container-widget-width,var(--spacer-size));--align-self:var(--container-widget-align-self,initial);--flex-shrink:0}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container,.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer,.e-con>.elementor-widget-spacer>.elementor-widget-container,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer{height:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner{height:var(--container-widget-height,var(--spacer-size))}
P95 BV and Ampersand Capital Partners join forces to establish first in class vaccine CRO

Leuven, Belgium, May 2nd, 2023 – P95 BV, a global provider of epidemiology and clinical research solutions with a focus on vaccines and infectious diseases, today announced a majority recapitalization by Ampersand Capital Partners, a private equity firm specializing in growth equity investments in the life science and healthcare sectors. Ampersand’s investment will support the growth of P95’s core business and fund the expansion of its CRO services supporting the vaccine and infectious disease end-markets. As part of the transaction, P95 also announced the appointment of Benoit Bouche as Chairman of the company’s Board of Directors. Dr. Bouche has spent his entire career in the pharmaceutical outsourcing industry as an entrepreneur, advisor, and investor. Benoit previously served as President and CEO of Nexelis, a former Ampersand portfolio company.
P95 was founded in 2011 by CEO Thomas Verstraeten and is a leader in sourcing, analyzing and reporting epidemiological data to assist public health agencies, research organizations and pharmaceutical companies in improving access to safe and effective vaccines. The company’s services include late stage observational and clinical studies, pharmacovigilance, literature reviews, and related consulting work. The company’s unique capabilities in primary study development, presence in key infectious disease geographies, and leadership of the EMA-endorsed COVIDRIVE project have established P95 at the forefront of observational research in vaccine and infectious disease therapeutic development. Dr. Verstraeten and the current P95 management team will continue their current executive leadership roles and remain shareholders of P95 alongside Ampersand.
Thomas Verstraeten, CEO and Founder of P95, stated “P95 is excited to have the support of Ampersand as we pursue our vision to be the world’s favorite global research partner in support of vaccine and infectious disease therapeutic development and use. We believe Ampersand’s resources and expertise will enable our team to accelerate growth while expanding the scope and depth of clinical support services we provide to vaccine stakeholders across the globe. This will help fulfill our purpose to bring better health for everybody, everywhere.”
Benoit Bouche, incoming Chairman of P95, commented “P95’s unique and recognized expertise has enabled the business to build a strong position in the pharma services ecosystem. As observational studies play an increasingly critical role in vaccine development, Thomas and his team have established P95 as a leader in this field. Together, we see substantial opportunity for development of the core epidemiology offering and expansion into complementary CRO offerings for vaccine and infectious disease clients.”
Eric Lev, General Partner at Ampersand and who together with colleague Hidde Van Kerckhoven will also be joining the P95 Board, added, “P95 is another great fit in Ampersand’s strategy of supporting pharma services companies with leadership positions based on differentiated scientific expertise. We believe P95 is poised for continued growth due to its unique service offering, global footprint, and broad customer base spanning the entire vaccine ecosystem. We look forward to partnering with Tom and his team.”
About P95 BV
Founded in 2011, P95 is a global company headquartered in Leuven (Belgium) and with local and regional offices located in Rotterdam (the Netherlands), Bogota (Colombia) and Bangkok (Thailand). P95 delivers cutting-edge expertise in epidemiology and pharmacovigilance. The P95 team is comprised of epidemiologists, pharmacovigilance specialists, biostatisticians, data scientists, medical writers, IT engineers and study managers, located in over 17 countries. P95 projects are largely focused on a broad range of infectious diseases and vaccines, and the company also offers customized services upon request. Additional information about P95 is available at www.p-95.com.
About Ampersand Capital Partners
Founded in 1988, Ampersand is a middle market private equity firm with more than $3 billion of assets under management dedicated to growth-oriented investments in the healthcare sector. With offices in Boston, MA and Amsterdam, Netherlands, Ampersand leverages a unique blend of private equity and operating experience to build value and drive long-term performance alongside its portfolio company management teams. Ampersand has helped build numerous market-leading companies across each of the firm’s core healthcare sectors. For additional information, visit www.ampersandcapital.com or follow us on LinkedIn.