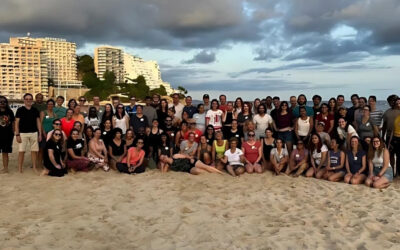In April 2025, P95 proudly opened two new offices on the African continent, in Kenya and Ghana, reinforcing regional impact and collaboration
P95 Transforming Exercise into Charitable Impact
At P95, we believe in the power of movement – not just as a means to personal health, but as a catalyst for global change. Our Strava group, an initiative that promotes health and fitness within the team, has successfully translated the time logged over the last year into substantial donations for three charities that make a real difference.
Laurence De Moerlooze Joins P95 as Chief Medical Officer
P95 BV, an Ampersand Capital Partners portfolio company, is proud to announce the appointment of Laurence De Moerlooze as Chief Medical Officer (CMO).
Celebrating P95’s Anniversary: From our Humble Beginnings to a Global Full-Service CRO
As we approach our 13th anniversary on June 16, we pause to celebrate important milestones and extend our deepest gratitude to our staff, partners and clients who have been part of our growth journey!
P95’s Journey From Epidemiology Consulting to Comprehensive CRO
In a recent interview for Ampersand Capital Partners, our CEO, Thomas Verstraeten, and Chairman, Benoit Bouche shared pivotal insights into P95’s growth from an epidemiology consulting into a global comprehensive Contract Research Organization (CRO).
Almost one year from P95’s partnership with Ampersand Capital Partners, the article dives deeper into P95’s expanding global reach, client-focused capabilities, and commitment to global health that bring our company to the forefront.
P95 welcomes John O’Brien as Chief Business Officer
We are excited to welcome John O’Brien as our new Chief Business Officer! John has a proven track record of driving commercial success and building strategic partnerships, bringing a wealth of experience to our team.
COVIDRIVE expands its scope of studies and becomes id.DRIVE: new European partnership for burden and effectiveness studies on infectious diseases
id.DRIVE is a Public-Private-Partnership, which consists of a unique consortium of pharmaceutical companies, public health institutes and research groups who are joining forces to monitor infectious diseases and effectiveness of related pharmaceutical products in real-life in Europe. This is achieved by several pharmaceutical companies co-funding a research network of hospitals.
Vaccination Programmes Book On Epidemiology, and Monitoring
Our P95 colleague Kaatje Bollaerts is excited to announce that the book ‘Vaccination Programmes | Epidemiology, Monitoring, Evaluation’ by Susan Hahné, Kaatje Bollaerts, and Paddy Farrington is now available to all to view and use online through a new open-access grant provided by CEPI.
P95’s fundraising initiatives
As actions speak louder than words, from an internal fundraising initiative, P95 made multiple donations in 2023 that will help charities across the globe.
international infectious disease observatory: A Joint effort from P95 and LOGEX
Healthcare analytics provider LOGEX and P95, a company specialized in epidemiology of infectious diseases, have signed...
Official Opening of P95 Thailand
Almost two years after expanding its operations to Latin America with the creation of P95 Latina, our company has...
P95 annual retreat in Mallorca
At the beginning of October, we held our Annual Retreat in beautiful Mallorca. Around 100 employees were present! This...
Take a tour of our new P95 Latina office
We're excited to share a quick tour of our new P95 Latina office in Bogotá, Colombia, which opened in August 2022. The...
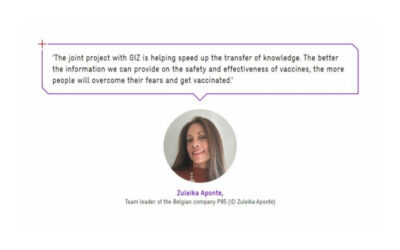
P95 recognized in the GIZ 2021 Integrated Company Report
The Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH's 2021 Integrated Company Report highlights the...
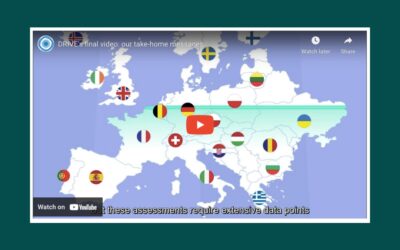
DRIVE project comes to an end: Key Outcomes & Insights
After five years, the Development of Robust and Innovative Effectiveness (DRIVE) project has come to an end. Set up in...
P95 celebrates its 11th birthday
We are celebrating the P95 birthday! On June 16h, 2022, P95 turned 11 years old. To everyone who has allowed our...
P95 Latina team meets in Bogota
On the week of May 16th to May 20th, some of the Epidemiology and Medical Writing team leads met the P95 Latina team...
“We saw in Latin America a hub of talented professionals in epidemiology and research”
Expanding to a different region is the dream of any growing company. For P95, the opportunity arose last year, when...
“I want people to know that P95 has an important role in the improvement of public health worldwide”
Tom Verstraeten, CEO of P95 shares about P95’s first nomination to the Trends Gazelles, recognizing the fastest-growing companies in Belgium
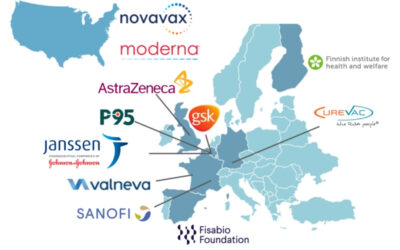
COVIDRIVE welcomes 3 new vaccine companies to the consortium
08/02/2022 – Three new vaccine companies (Moderna, Novavax and Valneva) have joined the COVIDRIVE consortium and officially become COVIDRIVE partners. Moderna, Novavax and Valneva will further contribute to the assessment of the effectiveness of COVID-19 vaccines in Europe to support the region’s public health response and address the vaccine companies’ regulatory obligations.
With the inclusion of these three newcomers, COVIDRIVE, which brings together public institutions, small and medium-sized enterprises and vaccine companies, has a total of 11 partners.