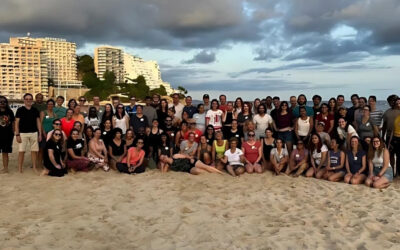Lyme Disease Publications
P95 has collaborated in a large review study about Lyme disease. This study has involved databases/surveillance and systematic reviews focusing on Lyme disease incidence, epidemiology, hospitalization, and clinical manifestations, among other outcomes, in different regions of the World.
P95’s fundraising initiatives
As actions speak louder than words, from an internal fundraising initiative, P95 made multiple donations in 2023 that will help charities across the globe.
international infectious disease observatory: A Joint effort from P95 and LOGEX
Healthcare analytics provider LOGEX and P95, a company specialized in epidemiology of infectious diseases, have signed...
Official Opening of P95 Thailand
Almost two years after expanding its operations to Latin America with the creation of P95 Latina, our company has...
P95 annual retreat in Mallorca
At the beginning of October, we held our Annual Retreat in beautiful Mallorca. Around 100 employees were present! This...
Take a tour of our new P95 Latina office
We're excited to share a quick tour of our new P95 Latina office in Bogotá, Colombia, which opened in August 2022. The...
P95 presents 5 posters at the International Conference on Lyme Borreliosis 2022
The P95 Lyme Team (Leah Burn, MPH, Jehidys Montiel Ramos, Aura Victoria Gutiérrez Rabá, Thao Mai Phuong Tran)...
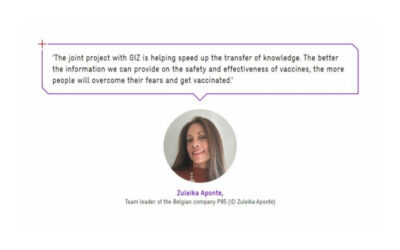
P95 recognized in the GIZ 2021 Integrated Company Report
The Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH's 2021 Integrated Company Report highlights the...
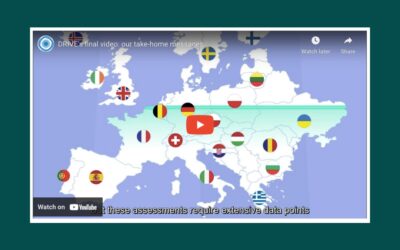
DRIVE project comes to an end: Key Outcomes & Insights
After five years, the Development of Robust and Innovative Effectiveness (DRIVE) project has come to an end. Set up in...
P95 celebrates its 11th birthday
We are celebrating the P95 birthday! On June 16h, 2022, P95 turned 11 years old. To everyone who has allowed our...
P95 Latina team meets in Bogota
On the week of May 16th to May 20th, some of the Epidemiology and Medical Writing team leads met the P95 Latina team...
“We saw in Latin America a hub of talented professionals in epidemiology and research”
Expanding to a different region is the dream of any growing company. For P95, the opportunity arose last year, when...
“I want people to know that P95 has an important role in the improvement of public health worldwide”
Tom Verstraeten, CEO of P95 shares about P95’s first nomination to the Trends Gazelles, recognizing the fastest-growing companies in Belgium
Phase 2b/3 trial HERALD: CVnCoV SARS-CoV-2 mRNA vaccine CANDIDATE
Additional safe and efficacious vaccines are needed to control the COVID-19 pandemic. We aimed to analyse the efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate.
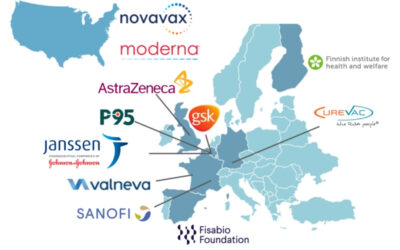
COVIDRIVE welcomes 3 new vaccine companies to the consortium
08/02/2022 – Three new vaccine companies (Moderna, Novavax and Valneva) have joined the COVIDRIVE consortium and officially become COVIDRIVE partners. Moderna, Novavax and Valneva will further contribute to the assessment of the effectiveness of COVID-19 vaccines in Europe to support the region’s public health response and address the vaccine companies’ regulatory obligations.
With the inclusion of these three newcomers, COVIDRIVE, which brings together public institutions, small and medium-sized enterprises and vaccine companies, has a total of 11 partners.
P95 nominated for the Trends Gazelles Flemish Brabant 2022
P95 is one of the nominees for the 2022 edition of Trends Gazelles Flemish Brabant. Trends Gazelles award aims to distinguish the fastest-growing Belgian companies over the past 5 years.
P95 appoints Henk Hoornaert as Chief Commercial Officer
P95 has appointed Henk Hoornaert as Chief Commercial Officer (CCO). After two years of accelerated growth, this appointment will strengthen P95’s structure and improve business development, marking a further step towards a more stable and mature organization.
WHO global vaccine safety multi-country collaboration project on safety in pregnancy
Standardized case definitions strengthen post-marketing safety surveillance of new vaccines by improving generated data, interpretation and comparability across surveillance systems.
Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 : A phase 1 randomized clinical trial
We used the RNActive® technology platform (CureVac N.V., Tübingen, Germany) to prepare CVnCoV, a COVID-19 vaccine containing sequence-optimized mRNA coding for a stabilized form of SARS-CoV‑2 spike (S) protein encapsulated in lipid nanoparticles (LNP).
Largest ever Lassa fever study expands to more countries in West Africa
Largest study to provide a more accurate assessment of the incidence of Lassa fever infections expands in West Africa