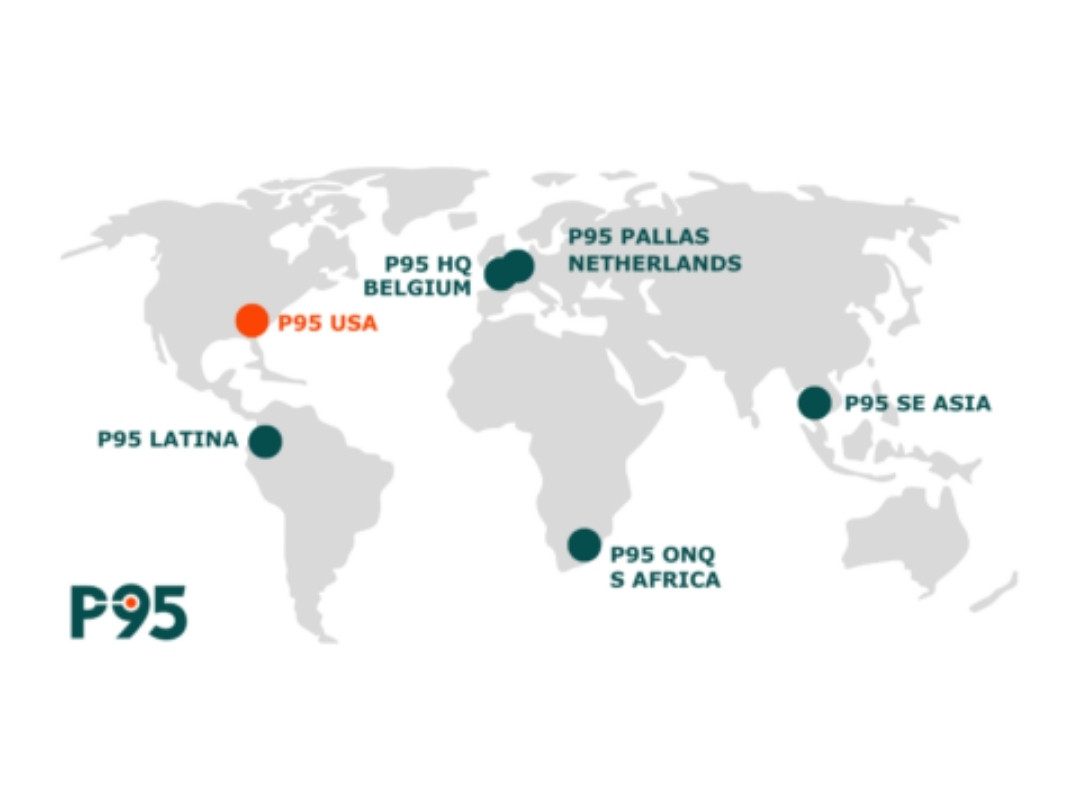
P95, a leading global provider of clinical and epidemiology services focused on vaccines and infectious diseases, has opened its first office in the USA. Based in the Research Triangle Park in North Carolina, the largest research park in the USA, this expansion underlines P95s commitment to delivering high-quality research solutions to our clients and partners across the globe.
The North Carolina USA office joins P95’s regional hubs in Europe, Africa, Southeast Asia and Latin America with over 200 experts in clinical operations, epidemiology, pharmacovigilance, data management, and biostatistics working together across continents and time zones. Together they will continue to provide first-class customized solutions covering clinical studies phase I-IV, epidemiology and RWE, and scientific and clinical development consulting.
“This is a significant milestone in our growth and development as a global contract research organization. This expansion will allow us to better serve our clients and partners in the region, as well as to leverage the opportunities and resources that North Carolina offers as a leading biotech and pharma hub. We look forward to building long-term relationships and delivering excellence in research in the USA and beyond.” – Thomas Verstraeten, P95 Chief Executive Officer
P95 is proud to be a truly global CRO, with the ability to offer high-quality services tailored to the local needs and regulations of each country where it operates. P95’s commitment to excellence in research is driven by its passion for vaccines and infectious diseases and its dedication to advancing public health worldwide.





0 Comments