innovative solutions, impactful outcomes.
P95 provides end-to-end, high-quality clinical trial services for vaccines and infectious diseases tailored to your specific needs.
With a shared goal to improve global access to safe and effective vaccines and infectious disease treatments, we leverage our experienced teams, over 1000 clinical trials, and an established site network to deliver customized solutions across all phases of clinical research.
End-to-End Trial Management
Global Expertise with Local Insights
High-Quality Results Aligned with Regulatory Standards
p95: Comprehensive CLinical Trial Support for Vaccines and Infectious Disease
Find out more
what
where
how
why us
what we do
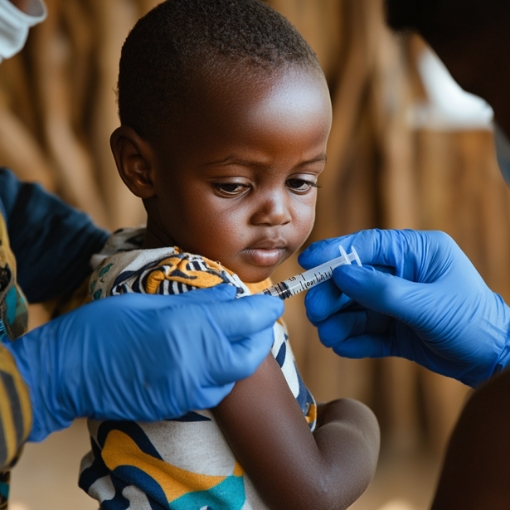
Vaccine development for emerging infectious diseases is key to improving global health, and we believe it’s possible with the right expertise in global clinical operations.
At P95, we apply over 25 years of clinical research expertise and experience to offer comprehensive clinical trial services for vaccines and infectious diseases. We guide projects from early to late phase trials and aim to consistently provide high-quality results that meet regulatory and global standards.
Our committed global team of over 500 professionals, including over 100 project management and clinical operations experts, delivers high-quality, flexible solutions to meet your unique research needs.
Our global reach extends to a network of more than 400 specialist vaccine and infectious disease clinical sites worldwide, ensuring that we can support the full study lifecycle or provide specialized consultancy based on your requirements.
Clinical trial management services for vaccines in LMICs
Our expertise in conducting infectious disease and vaccine trials in low- and middle-income countries means we can support clinical drug development in a variety of settings around the world, including Africa, Latin America, and South-East Asia.
Our local teams, combined with our site networks and local partners in these regions enable us to reach study participants in far corners worldwide. Uniquely positioned to harness the integration of epidemiology with clinical operations, we ensure the successful delivery of your clinical trial in the right countries and at the right sites.
Partner with a CRO specializing in vaccine development
Accelerating vaccine development to advance global health requires strong partnerships. As a CRO with expertise in managing infectious disease clinical trials and the vaccine development process, P95 provides the clinical trial solutions you need to make a difference.
We offer unique epidemiological expertise to support study design and site selection. Our services extend to feasibility analysis, protocol development, site management, participant engagement, pharmacovigilance, data management, biostatistics, and more. We also manage complex logistics, vendor selection, and ensure audit readiness throughout the trial.
Whether you need comprehensive trial support or specific services, P95 provides trusted expertise and reliable results to help bring innovative treatments vaccines and infectious diseases to market faster.

differentiators
- The range of a multinational with the flexibility and dedication of a boutique: we combine multi-phase international experience with the advantages of a more focused approach, with tailored solutions, personalized services and faster decision-making
- Dedicated experts in infectious disease vaccine trials: with experience across 1000 clinical trials, 75% of which were in vaccines and infectious diseases, we combine our operational capabilities with scientific excellence
- Expertise in phase I, II, III and IV studies: we provide comprehensive and scalable support from early-phase trials to post-marketing study designs
- Local and experienced teams: with our headquarters in Belgium, many of our team members are based around the world and can be available to site staff in person or within the same time zone
- Extensive site network: we can reach participants in a variety of settings worldwide
- Flexible solutions and a personalized approach, customized around the individual needs of each of our clients: we are used to adapting resources to project needs and can tap into a pool of experienced and qualified team members
- Access to functional expertise: our flexible model and depth across functional services, means we can offer the complete A-Z solution for any study, or any combination of services
- Project leadership and accountability: our flat management structure gives you access to our executive management team for oversight and issue escalation
services
Study design and protocol development
Site selection and feasibility
Epidemiological feasibility
Start-up, IRB/EC and RA submissions
Clinical project management
Site management and clinical monitoring
Site capability and capacity building
Data management
Vendor management
Medical monitoring
Biostatistics
Quality assurance
Safety and pharmacovigilance
Medical writing and publishing
Sample management and logistics
Request more information
Use the form below to contact us.